Air quality impacts found during the manufacture of ceramics

The ceramics industry is responsible for a great number of practical and decorative materials. From bricks used in constructing buildings, to fine china tea sets, the range of applications provided by silica manufacturing is impressive. But this range also implies a great demand for ceramic products, and a great number of ceramic manufacturing facilities around the world.
Each factory possesses their own exhaust stacks, used for emitting the hot gases produced when firing kilns. Thanks to both these gas emissions and the large amounts of particulate matter that can pollute the workspaces of ceramic plants, air quality can be a major concern for both workers and the environment surrounding ceramic factories. This article will explore the process of manufacturing brickwork and ceramics, while discussing potential emission sources, their environmental dangers, and how the industry can adapt to mitigate them.
From Clay to Teacup
Ceramics, and the families of ware that fall under that category, are produced from the preparation, shaping, and firing of clay. Clays are a family of minerals that develop plasticity when wet and become brittle and hard once dried or fired.
The bonding structure in source clay particles is generally ionic (The Clay Minerals Society, 2020). Since there are no free electrons in the material, this means that properly treated clay forms a strong but brittle solid that doesn’t easily conduct heat or electricity. These properties allow ceramics to be used in such a wide variety of applications.
There are several different types of ware produced from clay, depending on the quality of the material. The main technique for creating ceramic objects is an ancient practice that has been refined by factories over the centuries. Different products – brick, china, stoneware, tile, and pottery – still follow the same basic recipe. The below figure shows a general flow diagram of the steps involved in shaping raw clay into a final product.
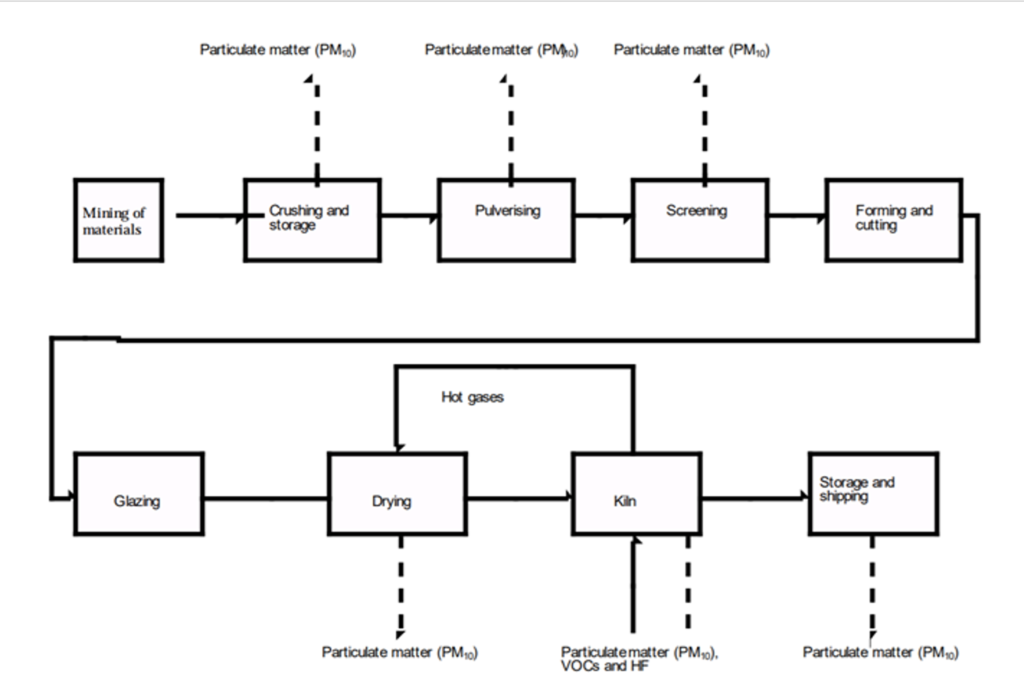
Flow diagram of the ceramic manufacture process (in this case brickworks). Dashed lines indicate steps where air quality may be impacted. (National Pollutant Inventory, 1998)
First, the clay must be extracted, crushed, and wetted to form slip. This is a malleable material that can be shaped into the desired vessel. For brickwork, this shaping process is made uniform through machinery, but for more complicated decorative plates or sculptures, the process takes a bit more time. You may be familiar with the concept of a potter’s wheel, used for throwing the slip symmetrically by hand. After creating one master sculpture with this technique, a mould can be used to quickly reproduce the design. This allows mass production to easily create many similar but complicated shapes (National Pollutant Inventory, 1998).
After the soft clay has been shaped, the still malleable ware is coated in a glaze. This ensures a waterproof and shiny final product after the wares have been fired. After the glazing, the ware is dried, usually with exhaust hot gasses from the current batch being fired (National Pollutant Inventory, 1998). This helps eliminate any unwanted cracks in the final product. Pottery that is not evenly dried before firing can have the dry clay pull away from the moist clay as the ware shrinks in the kiln (Scott, 2020).
Finally, it’s time to send the batch of would-be ceramics through the fire and flames. The ware passes through the kiln, a large oven that can reach extreme temperatures. Low fire kilns used for stoneware average around 1000 ̊C, while high fire porcelain kilns reach temperatures of up to 1340 ̊C (Soul Ceramics, 2020). Batches can remain in these environments for several hours before emerging at the end of the process as shiny, sturdy ceramics.
PM10 and Silicosis
In the figure presented earlier, you may have noticed dashed lines emerging from certain processes. These represent common emissions into the surrounding atmosphere during ceramic production. For many of these steps, a major concern is the release of particulate matter with particle diameters less than 10 microns (PM10).
Clays contain large amounts of crystalline silica. As the dry clay is crushed or stored, fine particles of silica dust can become airborne. Scraps of pottery left to dry on the floor can later dry and become pulverised. This can further contaminate the airspace. Although silica dust is not hazardous by skin contact or ingestion, chronic inhalation can lead to respiratory problems like silicosis, or “potter’s rot” (Princeton University, 2020).
Depending on the quality or type of clay, further respiratory hazards may be present. In porcelain manufacture, a major component used is kaolin – a malleable white clay. Inhalation of kaolin dust can lead to a mechanical clogging of the lungs.
Impurities in talc, a component added to wet clay to create slip, can also contaminate the surrounding workspace. These materials include asbestos and asbestos-like materials, which have been shown to cause serious health issues if inhaled or ingested.
Other substances that can be dispersed into the workspace include sand, perlite, ground firebrick and vermiculite. These are added to the clay to modify its properties and they all contain free silica. This makes them toxic to inhale.
With so much hazardous particulate matter floating around, ceramics manufacturers need to take steps towards keeping their workers safe from poor indoor air quality. Raw materials and finished ware should both be stored in separate rooms to product lines, as well as kept on racks that avoid dust accumulation. Scheduling clay mixing to the end of the day so that airborne dust can settle overnight when nobody is around is another simple strategy of minimising inhaled dust. These methods, when combined with regular use of dust cleaners and wet mopping of floors can help minimise the hazard of breathable particulate matter.
A Heavy Colour to the Air
If you remember our article on paints and material coatings, we mentioned the volatile solvents that coatings can emit into the atmosphere after application. The glazes in ceramic manufacture also volatilise when they are fired. Toxic materials used to produce a certain colour or texture in the ware can evaporate under the extreme temperatures, often leaving the facility through an exhaust stack. These compounds mix with the atmosphere and can be carried great distances to contaminate the surrounding area.
Glazes are made up of silica, colourants, and fluxes. Fluxes are materials that lower the usually high melting point of the other glaze materials such that the glaze will partially, or entirely, liquify inside the kiln. This creates the desired protective and decorative shine on the final ceramic product. Lead was one of the most common fluxes until its poisonous qualities were widely known. These days lead based fluxes are often replaced with boron and zinc fluxes (Princeton University, 2020).
In terms of air emissions, the cocktails of metal oxides that make up the colourants of glazes are also a major concern. Iron (red), copper (green), and cobalt (blue) are only a few common examples of heavy metals whose derivative compounds can bring artistic expression to ceramic wares. However, these colourants can contaminate the exhaust of the kiln, and impact the surrounding environment by spoiling the air or depositing onto nearby sources of water and soil.
The heavy metals found in flux and colourants are systemic toxicants, meaning that they interfere with the entire body. Although in small doses they are vital to several biochemical and physiological functions, excess amounts of these metals can lead to cell and tissue damage. They are known to induce adverse health effects in humans, such as cardiovascular diseases, developmental abnormalities and various types of cancer (Tchounwou, Yedjou, Patlolla, & Sutton, 2012). If these toxic particles are being discharged into the atmosphere with every batch of decorative pottery, mitigation strategies must be employed to minimise this pollution for the good of local communities.
Mitigation
Each ceramic manufacturing process will be slightly different depending on the ware being made, but there are still common mitigation strategies that can be employed to help minimise air emissions both indoors and outdoors. Stack monitoring and testing should be used for all ceramic kilns, with a focus on hazardous particulate matter, heavy metal concentrations and toxic gases formed by any secondary compounds mixed with the clay before being fired. Inside the manufacturing plants, appropriate measures against airborne silica dust will help protect workers from falling ill through inhalation. Finally, swapping out hazardous raw materials – such as lead fluxes, low quality clay, and easily volatilised glazes – can help innovate the industry towards a safer future for the atmosphere.
There is no doubt that ceramics are useful, beautiful, and hidden in more places than expected. Since the anthropogenic manipulation of these minerals will no doubt continue, we should do our best to ensure that we do not disrupt the rest of the planet and our own health when creating such practical and impressive devices.
References and Further Reading
Bartel, M. (2011, June 28). Hazards in Ceramics. Retrieved from Goshen College Art Department: https://www.goshen.edu/art/DeptPgs/Hazards.html
IBISWorld. (2017, April). Ceramic Product Manufacturing – Australia Market Research Report. Retrieved from IBIS World: http://vmwww2.ibisworld.com.au/industry-trends/market-research-reports/manufacturing/non-metallic-mineral-product/ceramic-product-manufacturing.html
National Pollutant Inventory. (1998). Emissions Estimation Technique Manual for Bricks, Ceramics, & Clay Product Manufacturing. Canberra: Environment Australia.
Princeton University. (2020). Ceramics. Retrieved from Princton University Environmental Health Safety: https://ehs.princeton.edu/health-safety-the-campus-community/art-theater-safety/art-safety/ceramics
Scott, S. (2020, February 26). The Clay Drying Process: Hints for Drying Pottery Evenly. Retrieved from Ceramic Arts Network: https://ceramicartsnetwork.org/daily/pottery-making-techniques/handbuilding-techniques/clay-drying-process-hints-drying-pottery-evenly/
Soul Ceramics. (2020). Guide to Kiln Temperature Ranges for Pottery. Retrieved from Soul Ceramics: https://www.soulceramics.com/pages/guide-to-kiln-temperature-ranges-for-pottery
Tchounwou, P., Yedjou, C., Patlolla, A., & Sutton, D. (2012). Heavy Metals Toxicity and the Environment. In A. Luch, Molecular, Clinical and Environmental Toxicology (pp. 133-164). Basel: Springer.
The Clay Minerals Society. (2020). Physical and Chemical Data of Source Clays. Retrieved from The Clay Minerals Society: http://www.clays.org/sourceclays_data.html













