
Hendrik Zwaardemaker, considered by many to be the father of olfactometry. (Wikipedia Commons)
How Odour Science Has Developed Over The Years
Newton famously said that if it seemed he could see further than others, it was because he stood on the shoulders of giants – a metaphor for appreciating the foundations laid by the great thinkers before him. This month, Air Environment celebrates seven years of delivering great air quality and odour advice. And although we have many more years of innovative science still to come, we thought that we would use this opportunity to look back in time at the birth of odour science itself and appreciate some of its great thinkers. This article explores the origin story of olfactometry, and how this relatively new science evolved to the form that we use today.
In the Beginning
Olfaction is an old sense, but a new science. The human nose has evolved into a spectacular instrument for interpreting the odorous molecules around it, and for a look at how it does this, see our piece on the anatomical processes involved. But classifying the large number of odours that the human nose can detect is a daunting task. A quantifiable study for classifying and characterising odours was the initial goal that birthed the field of olfactometry.
Initially, there were attempts to sort odours into a few broad categories. One of the earliest systems came from Carolus Linnaeus, a Swedish botanist better known for devising the two-part system we still use today for formally naming species. Besides coming up with terms like Homo sapiens, he also suggested a system of seven categories for odours: Champhoraeous, Musky, Floral, Pepperminty, Ethereal, Pungent and Putrid (Philpott, Bennet, & Murty, 2008). Although this list had some abstract categories, it was still an initial formal attempt at sorting the billions of possible odour characters detectable by humans.
This broad group of odour sorting bins evolved over the years. The Dutch physiologist, Hendrik Zwaardemaker (more on him later), revised it to nine groups. He expanded the list to: Ethereal, Aromatic, Fragrant, Ambrosic, Alliaceous, Empyreumatic, Mircine, Foul, and Nauseous (Philpott, Bennet, & Murty, 2008). Yet despite the widening of the net, the complexity of odours did not fit single categories easily. The three-dimensional odour prism by Hans Hemming in 1916, attempted to define a six pointed possibility space where any odour could be theoretically represented by a point enclosed within (Hemming, 1916). And although it was intriguing, the simplicity of the prism system did not hold up to empirical testing (Wise, Olson, & Cain, 2000) (Finger, 2001).
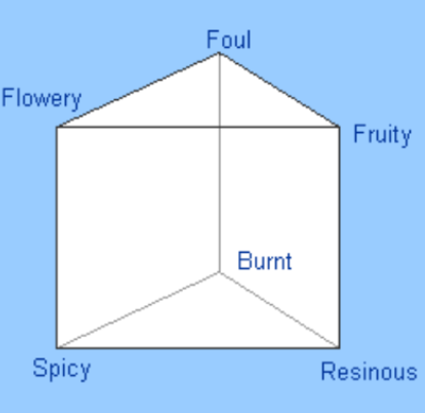
Hemming’s Odour Prism (Philpott, Bennet, & Murty, 2008)
But what kind of empirical testing was going on? The categories used to describe odours were revealing themselves to be problematic, vague, and generally unhelpful. They represented an attempt at formal objective characterisation, but odour characters depended on subjective experience. An odour may appear fragrant to you, but foul to somebody else. Instead, the world was finding less subjective methods to measure and define odours.
Let’s rewind the timeline back to the 19th century for a moment. While subjective categories were being formed, they lacked the understanding of neuroscience that we have today. During this time, a few prominent names published work that boosted the world’s fundamental understanding of olfaction. German physiologist Dr Gabriel Valentin published a large collection of works in his time, including a textbook on human physiology (Valentin, 1847) that helped set foundational knowledge of breathing systems and the physiology of our senses. Another school of study by J. Passy involved investigating the molecular structure of volatile odorant substances (Passy, 1892), trying to determine what chemical groups made odours occur.
But the experimental expert in olfactory studies at the time was Hendrik Zwaardemaker (we told you he’d be important), who had continued his work on odour categorisation and developed one of the first devices for determining the threshold of olfactory detection. Rather than focus on what name to call the odours that people detected, a more quantifiable method involved finding what concentration of a controlled odorant was necessary to trigger reactions from a subject.
Threshold Measurement Devices: The Heart of Olfactometry
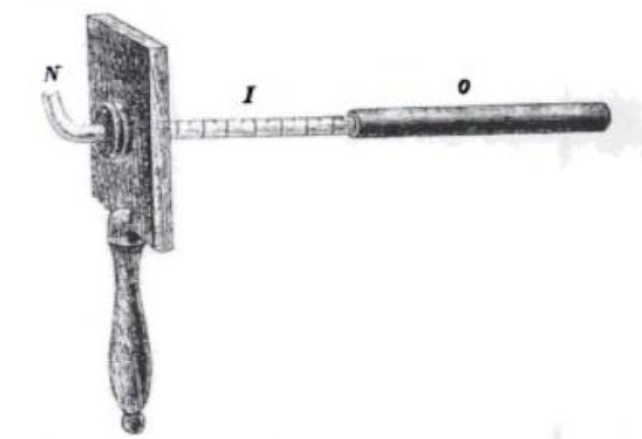
Zwaardemaker olfactometer as seen in the British Medical Journal in 1888 (Finger, 2001)
Devised in 1888 and credited as the first device of its kind, the Zwaardemaker olfactometer was a device that drew odorant into the nostril by way of a glass tube (Mateson, 1955) (Philpott, Bennet, & Murty, 2008). The length of the tube would determine the amount of odour being presented to the subject. With different presentations, the test subject’s detection threshold for the odorant could be quantifiably determined. This method of altering the concentration of odorous air until an odour could be detected would form the groundwork of olfactometry as we know it today.
Olfactometers are, put simply, dilution devices. Different levels of odorant diluted with clean air are presented in some form to a person. Typically, at the start of the presentation series, the odorant is undetectable. Yet as the test progresses, the sample becomes less and less diluted until the test subject (i.e. the odour assessor) can register its presence. This concept evolved from Zwaardemaker’s design into more complicated devices over the years. New methods of controlling air flow emerged once the technology for pumping and storing air also developed.
When air flow was not an easily available option, a collection of control samples with different odorant concentrations were used as presentations instead. A library of felt tip pens using liquid odorants instead of dye – playfully nicknamed “sniffin’ sticks” – could be used to determine the detection threshold of certain reference materials (Hummel, Sekinger, Wolf, Pauli, & Kobal, 1997). This collection of sniffin’ sticks could be used to test a subject’s ability to discriminate between different odours or different concentrations of the same odour when presented with multiple sticks. One would present three sticks to a subject in a random order, where only one contained the odorant at a given dilution. Presentations continued with increasing concentrations of odorant until the subject correctly discerned the odorant in two successive trials. This technique is still used today, often in medical applications like determining abnormalities in patient olfactory systems, but it is only practical for one person to be tested with the sticks at a time.

A Collection of Sniffin’ Sticks (MediSense, 2020)
These days, if we wish to determine the detection threshold of an unknown substance, dynamic flow olfactometry has become the standardised method for determining odour concentration. First published in 2003, the European Odour Standard EN13725 dictates the modern methods for odour testing around the world. Olfactometry standards are constantly being reviewed and revised so that they can provide the steps for best practice around the world (van Harreveld, Heeres, & Harssema, 2015). The Australian and New Zealand standard, AS/NZS4323.3 – Determination of odour concentration by dynamic olfactometry, was published in 2001 and was largely based on a draft version of EN13725.
The triangular presentations in dynamic olfactometry follow a similar process to the sniffin’ sticks’ presentation method. Instead of known concentrations of liquid odorant however, an odorous gas sample is diluted with clean air to a known concentration. It is then presented to a panellist with a choice of ports – one containing the sample and two containing clean air. The panellist then selects from which of the three ports they can detect an odour and records their certainty with the selection. The concentration of the odour increases over multiple presentations until the panellist is confidently correct twice in succession.
The olfactometers we have today are impressive pieces of dilution technology. Air Environment’s own odour lab houses Scentroid’s SS600 – a dynamic olfactometer that uses mass flow controllers to accurately provide a range of dilution steps for panels of up to six people at once. You can find out more about it here.
We also employ a portable, single port olfactometer, that uses a backpack reservoir of clean air and the venturi principle. The device draws in known amounts of diluting air through a series of critical orifices (i.e., tiny holes) set in a dilution plate. This compact and portable olfactometer allows one person in the field to immediately test concentration detection thresholds from either the ambient atmosphere, or direct from the odour source.
Other machines use a smaller range of choices, or only allow a few people to sit on the panel at one time. Nevertheless, the principle of altering dilutions until a detection is discovered remains the same as Zwaardemaker’s early concept.
Human or Non-Human Detectors?
Thanks to millions of years of evolution, our own olfactory system is the most sensitive odour detector that we have access to. For many years, odour evaluation by means of a panel of trained evaluators has been the main tool for the quantification and characterisation of odours. This is because when compared to machine counterparts, the human nose can detect a broader range of substances at extremely low concentrations (down to approximately 10-12g/mL (Wilson & Baietto, 2009)).
Our noses are also better suited for detecting mixtures or complex odours. Many machine detectors are exceptional at measuring the concentration levels certain known substances, like the toxic rotten egg gas Hydrogen Sulphide (H2S). Yet, a H2S monitor would be unhelpful in detecting the concentration threshold of odours that do not contain H2S. The number of receptors needed to detect obscure cocktails of sample gases can easily be found inside the human nose.
Another major reason that an odour panel of human perspectives are necessary for odour science is the heavy reliance on personal experience that comes with odour character and intensity. The subjectivity mentioned earlier means that a purely objective machine would only tell you the presence of a compound – not whether that compound is pleasant, matches a certain nuisance flavour, or if it becomes horrible when combined with another compound measured downwind. Since those who will be affected by odour are people, then it is in their best interest for people to be involved in the detection and measurement of nuisance odours.
The panellists are calibrated to a known reference material before being used to measure odour concentrations. This ensures that measurements are representative of the population average for detecting certain compounds. There are even a few studies into what makes up the perfect odour panellist (Bliss, Schulz, Senger, & Kaye, 1996) (Zarra, Reiser, Naddeo, Beligiorno, & Kranert, 2018), but the consensus remains that an effective odour panel includes a variety of backgrounds (for a greater ease in describing odour characters) and that each panellist remains consistent in their calibrated reference gas detection threshold.
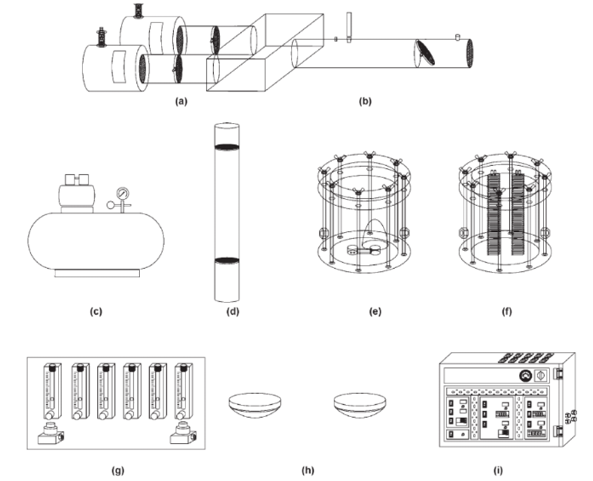
An olfactometer for observing mosquito behaviour. The device consists of: stimulus delivery chambers (a); a wind tunnel (b) and air compressor (c); an activated charcoal filter (d); humidity (e) and temperature (f) control chambers; gas flow (g) lighting (h) and electric (i) controls. (Omrani, et al., 2010)
Having said this, odour science does not rely entirely on calibrated human panels. Some product testing industries, like the makers of insect repellents, use olfactometry to determine the pleasantness of odours to other animals. The machines have a different design, but again they operate on the principle of presenting a panel of creatures with a choice of odorous air and clean air and observing their decisions or behaviours as dilutions vary.
An olfactometer used to determine whether mosquitoes are repelled by some product or not is shown on the left (Omrani, et al., 2010). Two hands are presented to the bugs in the centre of the device, one with insect repellent and one without. The direction that the bugs choose to fly indicates if they can detect the odour of food – and whether the product can mask that odour effectively. These machines have been in operation since 1907 and demonstrate how niche technology can be adapted to different industries (Omrani, et al., 2010) (Debboun, Frances, & Strickman, 2014).
Modern Machine Methods
Even though humans are more useful when it comes to measuring complex odour mixtures, recent technological advances have propelled the development of an electronic nose into the realm of possibility. Electronic nose instruments are another way to quantify odorous compounds without the use of a subjective panel and have been in development for the last few decades. (Gardner & Bartlett, 1994) (Wilson & Baietto, 2009) (Patel, 2014). The working principle involves an odour sample meeting an array of chemical sensors – each designed to pick up a specific compound. Together, the measurements form a kind of chemical fingerprint that can be referenced to a digital recognition library. This simulates the human brain recognising the odours that it detects from our array of olfactory receptors.
Electronic noses today make use of the large variety of gas sensor technologies that have been developed and refined over the years. These include conducting polymers, infrared and fluorescence sensors, metal oxide semiconductors, catalytic bead or field effect sensors and more (Wilson & Baietto, 2009).
The benefit of an electronic nose is its detachment from a living person. Toxic gases can now be tested for chemical fingerprints without putting the lives of odour panellists at risk. Electronic noses can also be used to give a “character” to odourless gasses, and help distinguish them from each other. Although the technology is still improving, the ability to have a customisable electronic chemical receptor array can find a home in industries like agriculture, manufacturing, safety, and environmental monitoring.
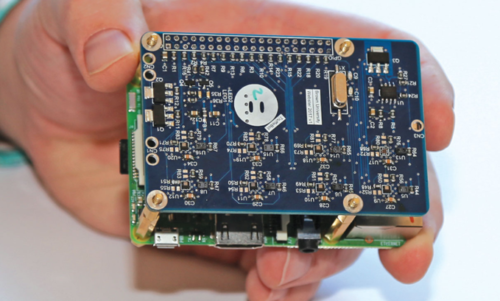
An example of an electronic nose – TruffleBot (Scudellari, 2018)
What’s Next for Odour Science?
The instruments and methods in odour science are constantly improving. Although the field is relatively young, novel olfactometer designs are still being developed. Combining olfactometry with chemical sensors like gas chromatography/mass spectroscopy techniques can help add odour character and concentration thresholds to the results of volatile chemical testing (Brattoli, et al., 2013). Olfactometers are also exploring the testing of odour mixtures, where multiple sample input channels can simultaneously expose panellists to concoctions of odorants (Burton, Wipfel, Guo, Eiting, & Wachowiak, 2019). In the ambient atmosphere, odorous concoctions are rarely isolated chemicals and a mixing olfactometer can provide concentration threshold measurements for substances that combine downwind of the source or evolve from chemical reactions in the atmosphere.
The work from more than one hundred years ago is also still being developed. Researchers are still trying to form an objective category system. These range from ranking a small population of monomolecular odorants with relevance to a limited set of descriptors (Koulakov, Kolterman, Enikolopov, & Rinberg, 2011), to removing subjectivity entirely and attempting to link chemical weight of a compound with how similar it smells to other compounds (Snitz, et al., 2013). Efforts so far have struggled to fully encompass the staggering number of possible odorants that the human nose can perceive, yet the field of study still marches on and attempts to further improve our classification of odours.
A famous quote from Alexander Graham Bell challenged the very idea of odour science. He claimed that “It is very obvious that we have very many different kinds of smells… But until you can measure their likenesses and differences you can have no science of odor”- (Bell, 1914). In the years since his dismissal, we would suggest that humanity have developed quite the impressive measure of olfaction. People have since produced involved categorisation systems and objective standard measurement techniques for threshold and intensity detection. Industries rely on a calculated method to quantify olfaction to help create more efficient products and protect the environment. If nothing else, it’s a good start to a young science. And perhaps one day, we can be the giants that future generations will look back to as innovators and experts in the science of smell.
References and Further Reading
Bell, A. (1914). Discovery and Invention. Press of Judd & Detweiler.
Bliss, P., Schulz, T., Senger, T., & Kaye, R. (1996). Odour Measurement – Factors Affecting Olfactometry Panel Performance. Water Science and Technology, 549-556.
Brattoli, M., Cistermino, E., Dambruoso, P., deGennaro, G., Giungato, P., Mazzone, A., . . . Tutino, M. (2013). Gas Chromatography Analysis with Olfactometric Detection (GC-O) as a UsefulMethodology for Chemical Characterization of Odorous Compounds. Sensors, 16759-16800.
Brattoli, M., deGennaro, G., dePinto, V., Loiotile, A., Lovascio, S., & Penza, M. (2011). Odour Detection Methods: Olfactometry and Chemical Sensors. Sensors, 11, 5290-5322. doi:10.3390/s110505290
Burton, S., Wipfel, M., Guo, M., Eiting, T., & Wachowiak, M. (2019). A Novel Olfactometer for Efficient and Flexible Odorant Delivery. Chemical Senses, 173-188.
Cozzutto, S., Pettarin, N., Barbieri, G., Licen, S., & Barbieri, P. (2018). Testing Performances of a Newly Designed Olfactometer. Chemical Engineering Transactions, 325.
Debboun, M., Frances, S., & Strickman, D. (2014). Insect Repellents Handbook. Boca Raton: CRC Press.
famousscientists.org. (2015, January 16). Carolus Linnaeus. Retrieved from Famous Scientists: https://www.famousscientists.org/carolus-linnaeus/
Finger, S. (2001). Origins of Neuroscience: A History of Explorations Into Brain Function. Oxford: Oxford University Press.
Gardner, J., & Bartlett, P. (1994). A brief history of electronic noses. Sensors and Actuators B: Chemical, 210-211.
Gralapp, A., Powers, W., & Bundy, D. (2001). Comparison of Olfactometry, Gas Chromatography, and Electronic Nose Technology for Measurement of Indoor Air from Swine Facilities. Swine Research Report, 2000, 28.
Hemming, H. (1916). Der Geruch. Leipzig: Barth.
Hummel, T., Sekinger, B., Wolf, S., Pauli, E., & Kobal, G. (1997). ‘Sniffin’ Sticks’: Olfactory Performance Assessed by the Combined Testing of Odor Identification, Odor Discrimination and Olfactory Threshold. Chemical Senses, 39-52.
Koulakov, A., Kolterman, B., Enikolopov, A., & Rinberg, D. (2011, September 15). In search of the structure of human olfactory space. Frontiers in Systems Neuroscience, 5, 1-8. doi:10.3389/fnsys.2011.00065
Mateson, J. (1955). Olfactometry: Its Techniques and Apparatus. Journal of the Air Pollution Control Association, 167-170.
Omrani, S., Vtandoost, H., Oshaghi, M., Shokri, F., Guerin, P., Ershadi, M., . . . Tirgari, S. (2010). Fabrication of an olfactometer for mosquito behavioural studies. Journal of Vector Borne diseases, 17-25.
Passy, J. (1892). L’odeur dans la série des alcohols. C. R. Soc. Biol., 447-449.
Patel, H. (2014). The Electronic Nose: Artificial Olfaction Technology. New Delhi: Springer India.
Philpott, C., Bennet, A., & Murty, G. (2008). A Brief History of Olfaction and Olfactometry. The Journal of Laryngology & Otology, 657-662.
Scudellari, M. (2018, October 26). Meet the E-Nose That Actually Sniffs. Retrieved from IEEE Spectrum: https://spectrum.ieee.org/the-human-os/biomedical/devices/meet-the-enose-that-actually-sniffs
Snitz, K., Yablonka, A., Weiss, T., Frumin, I., Khan, R., & Sobel, N. (2013). Predicting Odor Perceptual Similarity from Odor Structure. PLOS Computational Biology, e1003184.
Valentin, G. (1847). Lehrbuch der Physiologie des Menschen. Brusnwick: Friedrich Vieweg and Sohn.
van Harreveld, A., Heeres, P., & Harssema, H. (2015). A Review of 20 Years of Standardization of Odor Concentration Measuremet by Dynamic Olfactometry in Europe. Journal of the Air & Waste Management Association, 705-715.
Wilson, A., & Baietto, M. (2009). Applications and Advances in Electronic-Nose Technologies. Sensors, 5099-5148.
Wise, P., Olson, M., & Cain, W. (2000, August 4). Quantification of Odour Quality. Chemical Senses, 25(4), 429-443. Retrieved from https://academic.oup.com/chemse/article/25/4/429/342742
Zarra, T., Reiser, M., Naddeo, V., Beligiorno, V., & Kranert, M. (2018). A critical evaluation of the influence of different panel composition in the measurement of odour concentration by dynamic olfactometry. Chemical Engineering Transactions, 68, 1-6. doi:10.3303/CET1868001











